Introduction: BsAbs and CAR T-cell therapy have revolutionized the treatment landscape of patients with relapsed/refractory (R/R) LBCL. Regrettably, less than 40% of patients will attain durable responses, and determinants of response remain unclear. Our aim was to identify tumoral characteristics that can predict resistance to CAR T-cell therapy and BsAbs in LBCL patients.
Methods: We retrospectively collected clinical data and obtained pre-treatment formalin-fixed and paraffin-embedded specimens prior CAR T-cell and BsAb treatment from 56 LBCL patients from 4 centers. Targeted NGS was performed to identify variants and copy number variations (CNVs) across a custom gene panel of 200 genes. These include lymphoma related genes, and genes involved in tumor antigen presentation, B and T lymphocyte interaction, and cell death pathway. Variables assessing response where Overall Response Rate (ORR), Complete Response Rate (CRR), Progression-Free Survival (PFS), and Overall Survival (OS). To ascertain each gene's influence on these endpoints, a logistic model was used for ORR and CRR comparisons, while Kaplan-Meier and Cox methods were employed to analyze PFS and OS.
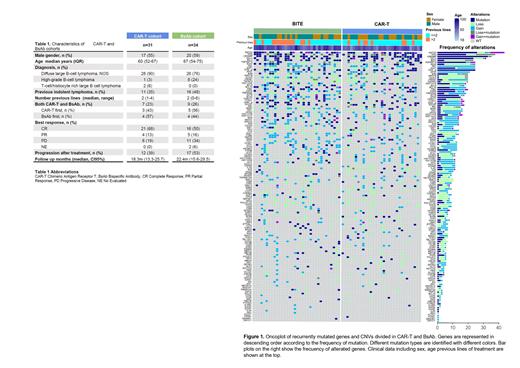
Results: A total of 56 patients were included in the study, 36 patients treated with CAR T-cell and 36 with BsAbs (16 patients received both). Samples were obtained for 31 patients prior to CAR T-cell therapy (CAR T-cell cohort) and 34 cases prior to BsAbs (BsAbs cohort). Baseline characteristics of the patient population are shown in Table 1. In summary, median age was 62 years, 57% were male, 42% had a previous indolent lymphoma and median previous lines were 2 (range 1-4). Best response included CRR in 59%, with a median PFS and OS of 13.6 (CI95% 5.98 - Not reached [NR]) and 29.5 (CI95% 15.7 - NR) months, respectively. Median follow-up for the full cohort was 18.7 months (CI95% 15.1 - 24). As per the CAR T-cell cohort, ORR and CRR were 81% and 68%, respectively. Median PFS and OS for CAR-T-cell cohort was 29.4 months (CI95% 5.98 - NR) and NR, respectively. Regarding the BsAb cohort, ORR and CRR were 66% and 50%, respectively. Median PFS and OS was 10 (CI95% 5.16 - NR) and 22.5 (CI95% 14.4 - NR) months, respectively.
For the full cohort, the most frequently altered genes, considering both mutations and CNVs, were KMT2D (66%), TP53 (60%), CREBBP (58%), IGLL5 (51%), REL (42%), EZH2 (38%), BCL2 (38%), TNFRSF14 (37%), SGK1 (32%) and KLHL6 (32%) (Figure 1). The most frequent CNVs were gains of 2p16.1 (34%), 3q28 (15%), 6p(14%), 11q (12%), 21q (12%), 3q (10%); and losses of 17p (32%), 6q (26%), 1p36.32 (25%), 13q14.2 (18%), 19p13.3 (18%), 4q35.1(16%), (10q23.31 (12%), 9p21.3 (10%).
Focusing on the CAR T-cell cohort (n=31), mutations and/or deletions in EP300 (7%, n=2)( p=0.01) and KLHL6 (n=2, 7%) ( p=0.04) were associated with an inferior PFS. In addition, mutations and/or deletions in KLHL6 ( p=0.02) and EP300 (n=3, 10%) ( p=0.05), and amplifications in MYC (n=3,10%) ( p=0.031) were associated with shorter OS. No differences in PFS and OS were oberved in patients with mutations and/or deletions in TP53 (n=19, 63%). Regarding RHOA patients with mutations or deletions (n=2, 7%) did not present an inferior PFS ( p=0.88) or OS ( p=0.52). The analyzed alterations did not show an impact on response rate.
In terms of patients who received BsAbs (n=34), we observed that mutations and/or deletions in TP53 (n=19, 58%) ( p=0.04) , RHOA (n=5, 15%) ( p=0.05) and GNAI2 (n=5,15%) ( p=0.02)were associated with aninferior PFS. Mutations and/or deletions in TP53 (p=0.04) , RHOA (p=0.009) , GNAI2 ( p=0.01) and CD274(PDL1)-PDCD1LG2(PDL2) (12%, n=3) ( p=0.01) were associated with an inferior OS. Regarding MYC, 8 cases were mutated (n=2, 6%), amplified (n=5, 15%) or both (n=1, 3%), but these alterations were not associated with an inferior PFS (p=0.46) or OS ( p=0.27). Again, no impact on response rate was observed for patients carrying alterations.
Conclusions: In our study, a pattern of genetic alterations predicting worse OS was observed in patients treated with CAR T-cells ( KLHL6 EP300 and MYC) and BsAbs (TP53, RHOA, PDL1, PDL2, GNAI2). This important information will aid in the development of targeted strategies to overcome resistance and improve treatment outcomes.
Disclosures
Iacoboni:Abbvie: Honoraria; Novartis: Consultancy, Honoraria; MSD: Honoraria; Miltenyi: Consultancy, Honoraria; Autolus: Consultancy; Janssen: Honoraria; Gilead Sciences: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; AstraZeneca: Honoraria. Carpio:BMS: Consultancy; Regeneron Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Novartis: Honoraria. Cabirta Touzón:BeiGene: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; AbbVie: Other. Barba:Jazz Pharmaceutical: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pierre-Fabre: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nektar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Costa:Abbvie: Consultancy, Honoraria; Roche: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Astrazeneca: Consultancy, Honoraria; Janssen: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal